Density of a body
What´s density? What´s it used?
If you can not see the applications of this page, you can download Java at: http://www.houspain.com/gttp/salsaj
The density is a magnitude used by the physics and chemistry to determine the amoun of mass contained in a given volume.
There are two types of densities:
- ABSOLUT DENSITY: the density, which measure the mass per unit volume and is usually known by density.
Therefore, to calculate the density of a body you have to consider the following formula:
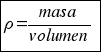 or that´s the same
or that´s the same 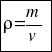
where –mass is the number of particles that make up the substance or body. The mass is measured in grams.
(What´s the difference between mass and weight of a body?)
–and the volume is the total number of scattered molecules.
The volume is in cubic units (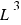 ).
).
- RELATIVE DENSITY: is the density that compares the density of a substance with the density of water.
Is calculated using the followingo formula:
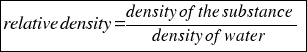 or that´s the same
or that´s the same 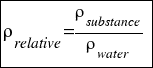
The density of water is 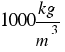 or that´s the same
or that´s the same 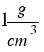
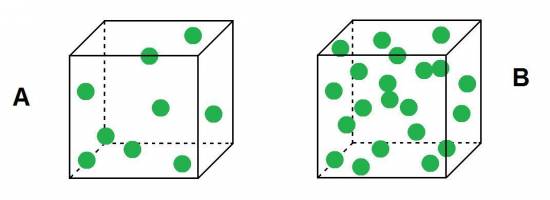
:?:Observes these two buckets, which belives it has greater density?
Because we know that density is the amount of mass contained in a given volume, it is easy to see that as the buckets are equal (have the same volume) and B in the cube is more number of particles (ie, higher mass), the density of the bucket B is greater than the density of the cube A.![]()
:?:Observe the following photograph of the globular cluster M13 or Hercules globular cluster:
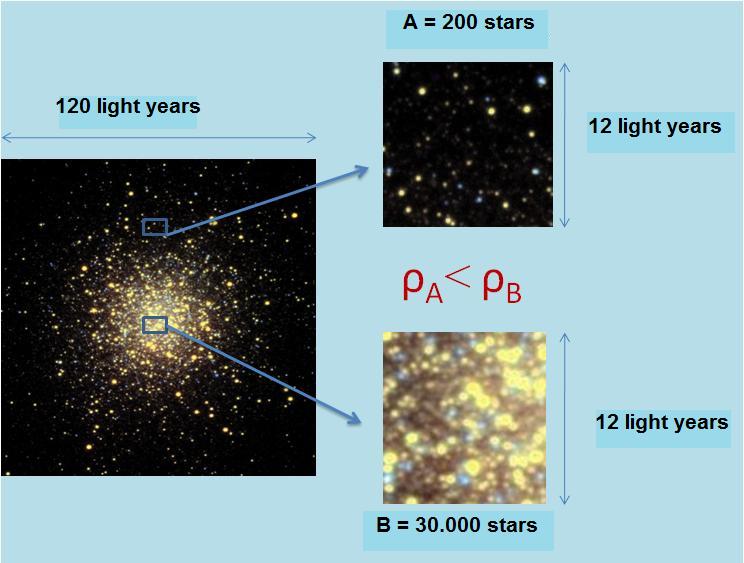
We take two regions of the cluster, A and B.
For the same volume (square of 12*12 light years), we see that the area A accommodates smaller number of stars that the area B .
Which region has the lowest density?
A volume equal to the two areas, area B contains the largest number of stars in zone A, therefore the density of A is less than the density of B.![]()
In nature, the matter can be presented in three different states: solid, liquid or gaseous.
Let´s go to look some examples of densities of various elements and substances most common and known to us.
Some solids are:
| Sodium | Diamond | Silicon dioxide | Cesium chloride | |
|---|---|---|---|---|
| densidad (g/cm³) | 0.97 | 3.5 | 2.6 | 3.99 |
Some liquids are:
| Water | Ethanol | Essential Oil | Petrol | |
|---|---|---|---|---|
| densidad (g/cm³) | 1 | 0.79 | 0.92 | 0.71 |
| Ozone | Carbon Dioxide | Air | Ammonia | |
|---|---|---|---|---|
| densidad (g/cm³) | 2.14 | 1.6 | 1.18 | 0.73 |
The data given in this section of gases, are data in normal pressure and temperature, ie:
pressure = 1 atmosphere
temperature = 0º = 273 K
Example
A ball of radius 51.6 cm is composed of 40% iron and the rest is aluminium.
a)What is the area and volume of the ball?
(What are the area and volume of a spherical body?)
b)What is the weight of the ball?
(What is the weight of a body?)
a) The area of any sphere is:
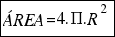
As the radius of the ball is R=15.6 cm, the area is:
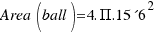 = 3058 square centimeters.
= 3058 square centimeters.
The volume of a sphere is equal to:
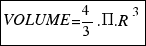
As the radius of the ball is R=15.6 cm, the volume turns out to be:
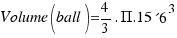 = 15902.4 square centimeters.
= 15902.4 square centimeters.
b) To calculate the weight of the ball we need to know the densities of the materials that comprise it.
The density of iron is: 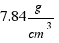
The density of aluminium is: 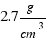
For the definition of density: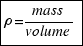 . Then the mass of the ball is:
. Then the mass of the ball is:
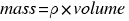 .
. 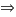 mass = (7.84×0.4 + 2.7×0.6)*15902´4 = 75759 grams
mass = (7.84×0.4 + 2.7×0.6)*15902´4 = 75759 grams
Therefore, for a body in the earth´s surface, where g = 9.8, the weight is:
weight = 75´759 x 9.8 = 742 Newton approximately.
- BUOYANCY
What is buoyancy?
The density of a body is related to the buoyancy: a substance will float on the other if their density is lower.
:?:What happens when we launch a paper airplane to water?

The density of the paper is 0.77 g/cm³ and the density of water is 1 g/cm³, therefore, at the begining the paper fleet becouse the density of the sheet is lower than the density of water.

After a short period of time, just need to soak the sheet and become a grater density (particles bind water and cellulose), the blade falls to the bottom because the takes a higher density than the water.
:?:What about wood, lead and water?
— The wood floats on water while the lead sinks into it, and that lead has greater density than water and the wood density is lower..
:?:What about wood, lead and gasoline?
— However if we have galoline instead of water, wood sink and lead in gasoline, as thes density of gasoline is lower than that of lead and wood.