Tabla de Contenidos
Calculating Moon´s density
What do you need?
- Paper and pen.
- You must know about:
If you can not see the applications of this page, you can download Java at: http://www.houspain.com/gttp/salsaj
Statement
What is the proportion of elements that shapes the Moon?
The Moon is formed mainly by the following elements:
OXYGEN, density: 1.4 g/cc
SILICON, density: 2.33 g/cc
ALUMINIUM, density: 2.7 g/cc
HIERRO, density: 7.86 g/cc
CALCIUM, density: 1.55 g/cc
MAGNESIUM, density: 1.74 g/cc
SODIUM, density: 0.97 g/cc
POTASSIUM, density: 0.86 g/cc
Make your proper composition of the Moon to obtein the real density of the Moon.
(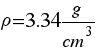 )
)
How can I resolve it?
What do we know?
We know the average density of the Moon, elements which forms the Moon and also we know the densities of all of them .
What do we have to find out?
You must calculate this one:
- The proportion of elements that forms the Moon. Observe, that radius will change if you take another choosen proportion.
Work out
- You can calculate it, if you make some combinations of the proportions, moving the buttons untill to obtein 100%.
- Use the formule that is known by us to calculate the average density.




